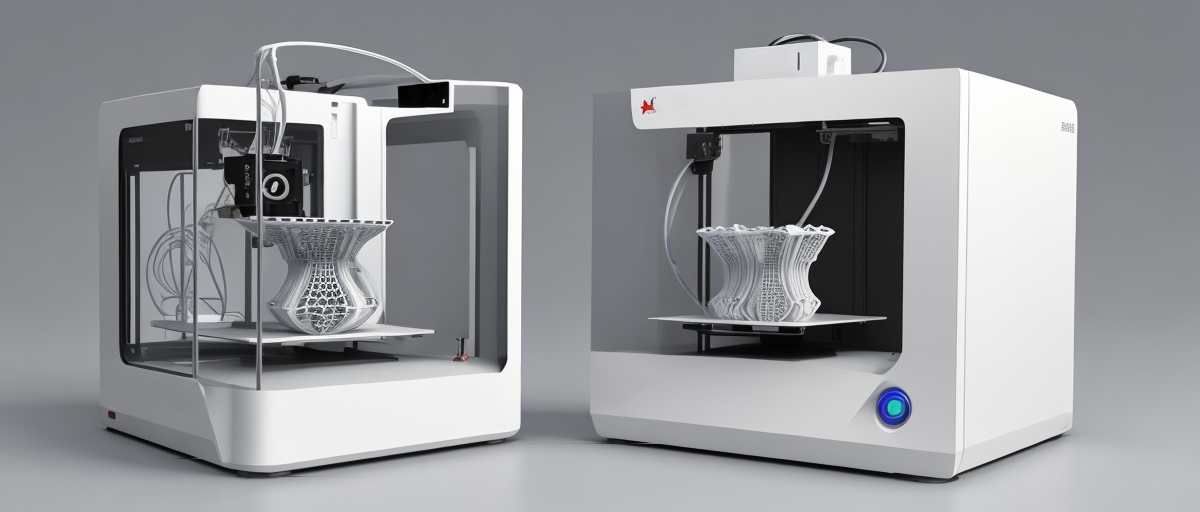
Tissue engineering is a rapidly growing field that aims to create artificial organs and tissues to replace or repair damaged biological structures. An important technological advancement driving this field is 3D printing, also known as additive manufacturing. This innovative technology allows for the precise fabrication of complex, three-dimensional structures by layering materials sequentially. 3D printing provides a promising avenue for developing functional tissues and organs tailored to the medical needs of individual patients.
Biomaterial Selection
The meticulous selection of biomaterials is pivotal to the success of 3D printing in tissue engineering. These materials must fulfill stringent criteria to function effectively within biological systems. Biocompatibility is important meaning the materials must not induce an adverse immune response when introduced into the body. They should support cell attachment, proliferation, and differentiation, enabling the cells to grow and form functional tissue.
The materials also need to be biodegradable, designed to gradually degrade at a rate commensurate with tissue formation. As the scaffold material degrades, the newly formed tissue should seamlessly take over the intended functions.
Common biomaterials can be categorized broadly into natural polymers, synthetic polymers, and hydrogels.
Natural polymers are derived from biological sources and are inherently biocompatible, often possessing bioactive properties that support cell functions. Examples include:
Collagen: primary structural protein in connective tissues, collagen provides an excellent framework due to its ability to support cell migration, attachment, and proliferation.
Gelatin: a derivative of collagen, gelatin is biocompatible and biodegradable, and its gel-forming properties make it a suitable candidate for 3D printing applications.
Alginate: extracted from brown seaweed, alginate gels in the presence of calcium ions, making it useful for creating cell-laden hydrogels. Its biocompatibility and ease of gelation are advantageous for fabricating tissue constructs.
Chitosan: derived from chitin, chitosan is known for its antimicrobial properties and excellent biocompatibility. It can be combined with other materials to enhance mechanical properties.
Synthetic polymers offer the advantage of tailored mechanical properties and controlled degradation rates. These materials are engineered for specific applications and include:
Polylactic Acid (PLA): a biodegradable thermoplastic derived from renewable resources like corn starch. PLA has good mechanical properties and is widely used in bone tissue engineering.
Polyglycolic Acid (PGA): known for its rapid degradation rate, PGA is often used in combination with other polymers to regulate the overall degradation profile of the scaffold.
Copolymers of PLA and PGA (PLGA): by adjusting the ratio of PLA to PGA, the properties of PLGA can be fine-tuned for specific applications, balancing strength, and degradation rate.
Hydrogels are networks of polymer chains that can absorb and retain large amounts of water. They are particularly useful in tissue engineering for encapsulating cells and delivering growth factors:
Water-Swollen Networks: these hydrogels mimic the extracellular matrix, providing a hydrated environment conducive to cell survival and tissue formation.
Cell-Laden Hydrogels: hydrogels can be loaded with living cells and bioactive molecules, facilitating the direct printing of functional tissue constructs. Their tunable properties enable the creation of complex, heterogeneous structures that closely mimic the natural tissues.
Each category of biomaterials offers unique properties that can be leveraged depending on the specific requirements of the tissue engineering application. By judiciously selecting and combining these materials, researchers can create scaffolds that closely replicate the intricate architecture and functionality of native tissues, paving the way for advanced regenerative therapies.
Bio-ink Development
The development of bio-inks is one of the most crucial aspects of 3D bioprinting, as these specialized inks must integrate both biological and material science principles to facilitate the creation of living tissues. Bio-inks are composed of living cells combined with biomaterials, and their formulation requires careful consideration of various properties to ensure successful printing and subsequent tissue growth.
One of the primary requirements for bio-inks is appropriate viscosity. Viscosity, or the measure of a fluid’s resistance to flow, must be finely tuned to balance the ease of extrusion from the printer nozzle and the structural integrity of the printed constructs. Low viscosity may result in poor shape fidelity and weak structures, while high viscosity can pose challenges in extrusion, risking cell viability due to shear stress. Optimizing viscosity is essential for preserving the integrity and functionality of the bio-ink.
Mechanical properties are another critical factor. The printed scaffold must possess sufficient mechanical strength to maintain its form and support the cells until the newly formed tissue can sustain itself. However, excessive rigidity can be detrimental to cell proliferation and differentiation. Therefore, the bio-ink must strike a balance between flexibility and strength, often achieved by adjusting the composition and crosslinking of the biomaterials used.
Stability is also important for the longevity and reliability of bio-inks. The bio-ink needs to maintain its properties under physiological conditions to support cell viability and function. This includes maintaining appropriate hydration levels, resisting premature degradation, and ensuring consistent mechanical properties over time. Stability ensures that the cells within the bio-ink remain viable, proliferate, and differentiate as needed to form functional tissue.
Viability is another fundamental aspect of bio-ink development. The process of bioprinting should not compromise the health of the encapsulated cells. Factors such as shear stress during extrusion, temperature, and exposure to light (in the case of photo-crosslinkable hydrogels) must be carefully managed to minimize cellular damage. Bio-inks are often formulated with additives like growth factors, hormones, and other bioactive molecules to enhance cell survival and promote desired biological outcomes.
The bio-ink must be tailored to mimic the native extracellular matrix (ECM) of the target tissue. The ECM provides structural and biochemical cues essential for cell behavior, including adhesion, migration, and differentiation. By incorporating ECM components such as collagen, fibrin, or hyaluronic acid into the bio-ink, researchers can create a more conducive environment for tissue development, closely replicating natural tissue dynamics.
Consideration in bio-ink development is the ability to incorporate multiple cell types and materials. Many tissues in the body are composed of various cell types working together in a complex architecture. Advanced bio-inks are being developed to allow the co-printing of different cellular and material components, enabling the creation of heterogeneous and functional tissue constructs. This multi-material approach can more accurately replicate the complex structures found in natural tissues.
Bio-ink development is increasingly focusing on customization and personalization. By tailoring bio-inks based on patient-specific cells and biomaterials, it is possible to create bespoke tissue constructs that are more likely to integrate successfully with the patient’s body, minimizing the risk of rejection and enhancing therapeutic outcomes.
The development of bio-inks is a multifaceted endeavor that requires a deep understanding of both materials science and cellular biology. By optimizing properties such as viscosity, mechanical strength, stability, and biocompatibility, and through the incorporation of bioactive molecules and customization, bio-inks can be engineered to support the successful bioprinting of complex, functional tissues and organs. This ongoing research and development hold immense promise for the future of regenerative medicine and tissue engineering.
3D Bioprinting Methods
Several bioprinting methods have been developed, each with unique advantages:
Extrusion-based Bioprinting: involves the continuous deposition of bio-ink through a nozzle. It is advantageous for printing large, heterogeneous structures and is relatively straightforward.
Inkjet Bioprinting: utilizes droplets of bio-ink, offering high precision and resolution. It is beneficial for high-throughput printing of small, complex structures.
Laser-assisted Bioprinting: employs laser pulses to transfer bio-ink droplets onto a substrate, providing precise control and minimal cell damage, though limited by higher costs and slower speeds.